Organic Chemistry – Some Basic Principles and Techniques
Which bond is more polar in the following pairs of molecules:
(i) H3C – H, H3C – Br
(ii) H3C – NH2, H3C – OH
(iii) H3C – OH, H3C – SH
(i) C – Br bond is more polar because Br is more electronegative than H.
(ii) C – O bond is more polar because O is more electronegative than N.
(iii) C – O bond is more polar because O is more electronegative than S.
Sponsor Area
Some More Questions From Organic Chemistry – Some Basic Principles and Techniques Chapter
How many chain isomers are possible for pentane?
Which characteristic is common to different isomers of a compound?
Name three alkanes which do not show chain isomerism.
Name the types of structural isomerism shown by alkanes.
Name the four main types of structural isomerism.
Write the three possible open chains of five carbon atoms
What types of structural isomerism is shown by the following pairs of organic compounds ?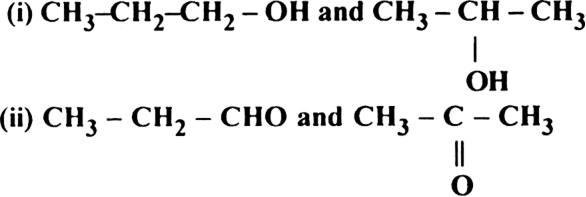
Write the tautomer of acetaldehyde and write its IUPAC name.
Draw the structure of the tautomer of phenol and write its IUPAC name.
Define ring - chain isomerism. Given one example.?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area