Sponsor Area
Atoms
The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?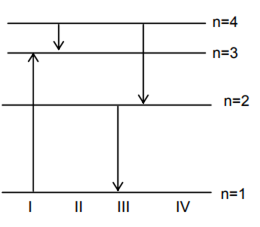
-
lll
-
IV
-
I
-
II
A.
lll
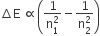
Some More Questions From Atoms Chapter
A proton and an electron travelling along parallel paths enter a region of uniform magnetic field, acting perpendicular to their paths. Which of them will move in a circular path with higher frequency ?
Draw graphs showing a variation of the photoelectric current with applied voltage for two incident radiations of equal frequency and different intensities. Mark the graph for the radiation of higher intensity.
Four nuclei of an element undergo fusion to form a heavier nucleus, with the release of energy. Which of the two — the parent or the daughter nucleus — would have higher binding energy per nucleon?
Which of the following transitions in hydrogen atoms emit photons of highest frequency?
The energy spectrum of β-particles [number N(E) as a function of β-energy E] emitted from a radioactive source is
Sponsor Area
Mock Test Series
Mock Test Series





