Sponsor Area
Nuclei
The transition from the state n = 4 to n = 3 in a hydrogen-like atom results in ultraviolet radiation. Infrared radiation will be obtained in the transition from
-
2 → 1
-
3 → 2
-
4 → 2
-
5→ 4
D.
5→ 4
IR corresponds to least value of 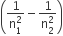 i.e. from Paschen, Bracket and Pfund series. Thus the transition corresponds to 5 → 4
i.e. from Paschen, Bracket and Pfund series. Thus the transition corresponds to 5 → 4
Some More Questions From Nuclei Chapter
When 3Li7 nuclei are bombarded by protons, and the resultant nuclei are 4Be8 , the emitted particles will be
The ‘rad’ is the correct unit used to report the measurement of
A photocell is illuminated by a small bright source placed 1 m away. When the same source of light is placed 1 /2 m away, the number of electrons emitted by photocathode would
Sponsor Area
Mock Test Series
Mock Test Series





