Sponsor Area
Atoms
State Bohr’s postulate to define stable orbits in hydrogen atom. How does de Broglie’s hypothesis explain the stability of these orbits?
Bohr's postulate:
An atom has a number of stable orbits in which an electron can reside without the emission of radiant energy. Each orbit corresponds, to a certain energy level.
Electrons revolve in a circular orbit. Centripetal force is provided by electrostatic force between electron and proton.
Given as,
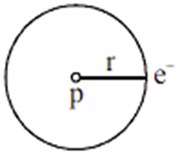
The motion of an electron in a circular orbit is restricted in such a manner that its angular momentum is an integral multiple of h/2π, Thus
3.
An electron may jump spontaneously from one orbit (energy level E1) to the other orbit (energy level E2) (E2 > E1); then the energy change AE in the electron jump is given by Planck’s equation
∆E = E2-E1 = hv
Where h = Planck’s constant.
And v = frequency of light emitted.
When the electron jumps from nth higher orbit to pth lower orbit it emits energy in form of the photon.
En - Ep = hv
According to de'Broglie hypothesis
Sponsor Area
Mock Test Series
Mock Test Series





