Question
An ideal gas is compressed to half its initial volume by means of several process. Which of the process results in the maximum work done on the gas?
-
Adiabatic
-
Isobaric
-
Isochoric
-
Isothermal
Solution
A.
Adiabatic
Given, ideal gas is compressed to half its initial volume i.e.,
Vo = V/2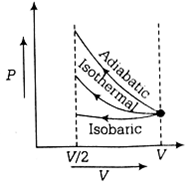
The isochoric process is one in which volume is kept constant, meaning that work done by the system will be zero ie.e Wisochoric = 0
As we know, work done on the gas = Area under curve i.e,
Wadiabatic > Wisothrmal > Wisochoric



