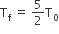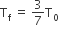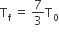Sponsor Area
Thermal Properties Of Matter
Two rigid boxes containing different ideal gases are placed on a table. Box A contains one mole of nitrogen at temperature T0, while Box B contains one mole of helium at temperature (7/3) T0. The boxes are then put into thermal contact with each other and heat flows between them until the gases reach a common final temperature. (Ignore the heat capacity of boxes). Then, the final temperature of the gases, Tf, in terms of T0 is
D.
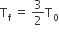
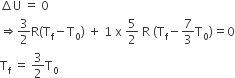
Some More Questions From Thermal Properties of Matter Chapter
If Cp and Cv denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively, then
A radiation of energy E falls normally on a perfectly reflecting surface. The momentum transferred to the surface is
Sponsor Area
Mock Test Series
Mock Test Series