Question
A lead bullet just melts when stopped by an obstacle. Assuming that 25% of heat is absorbed by obstacle, find the velocity of bullet. Initial temperature of bullet is 27°C, the melting point of lead is 327°C, the specific heat of lead is 0.03 cal/gm/K and latent heat of fusion of lead is 6 cal/gm.
Solution
Let m be the mass of bullet and v its velocity.
So,
Kinetic energy of bullet is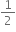 mv2.
mv2.
On stopping the bullet by obstacle, the kinetic energy of bullet is converted into heat.
25% of heat is absorbed by obstacle and remaining 75% of heat is absorbed by bullet.
Thus heat absorbed by bullet is,

This heat first increases the temperature of bullet and then melts it.
Therefore
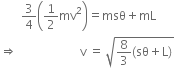
Here, we have
So,
Kinetic energy of bullet is
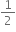 mv2.
mv2. On stopping the bullet by obstacle, the kinetic energy of bullet is converted into heat.
25% of heat is absorbed by obstacle and remaining 75% of heat is absorbed by bullet.
Thus heat absorbed by bullet is,

This heat first increases the temperature of bullet and then melts it.
Therefore
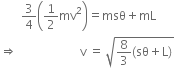
Here, we have
Specific heat of lead, s = 0.03 cal/gmK=30 x 4.2 J/kg/K
Latent heat of fusion, L = 6 cal/gm = 6000 4.2 J/kg
4.2 J/kg
Therefore, velocity of the bullet is given by,

Latent heat of fusion, L = 6 cal/gm = 6000
 4.2 J/kg
4.2 J/kgTherefore, velocity of the bullet is given by,
= 409.9m/s



