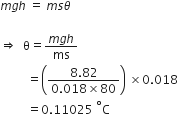Question
The water at the bottom of the Niagara Falls, which are 50 m high, should be warmer than that at the top. Explain why it is and calculate the temperature rise.
The heat capacity of 1 mol water (which weighs 0.018 kg) is 80 J/K.
The acceleration due to gravity is 9.8 m/s2.
Solution
Height of Niagara Falls, h = 50m
Let m be the mass of waterfall.
Decrease in potential energy of waterfall is,
U = mgh
U =
This potential energy is converted into heat.
Let θ be the rise in temperature.
Therefore,
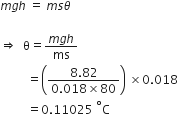
Let m be the mass of waterfall.
Decrease in potential energy of waterfall is,
U = mgh
U =

This potential energy is converted into heat.
Let θ be the rise in temperature.
Therefore,