Sponsor Area
Kinetic Theory
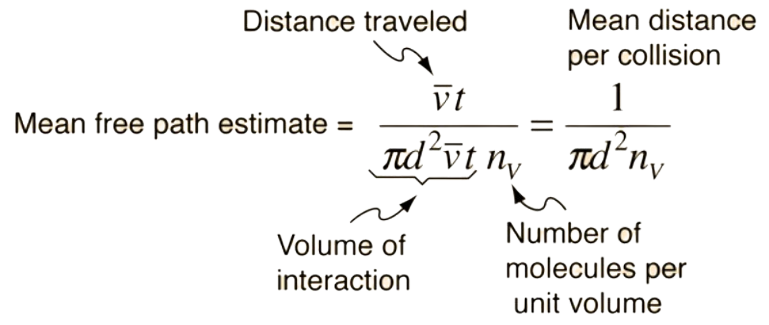
what do you mean by mean free path and write the formula?
In kinetic theory the mean free path of a particle, such as a molecule, is the average distance the particle travels between collisions with other moving particles. The formula still holds for a particle with a high velocity relative to the velocities of an ensemble of identical particles with random locations.
Some More Questions From Kinetic Theory Chapter
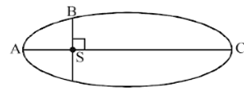
The kinetic energies of a planet in an elliptical orbit about the Sun, at positions A, B and C are KA, KB and KC, respectively. AC is the major axis and SB is perpendicular to AC at the position of the Sun S as shown in the figure. Then

Pressure of an ideal gas is increased by keeping temperature constant. What is the effect on kinetic energy of molecules?
Pressure of an ideal gas is increased by keeping temperature constant. What is effect on kinetic energy of molecules?
Sponsor Area
Mock Test Series
Mock Test Series





