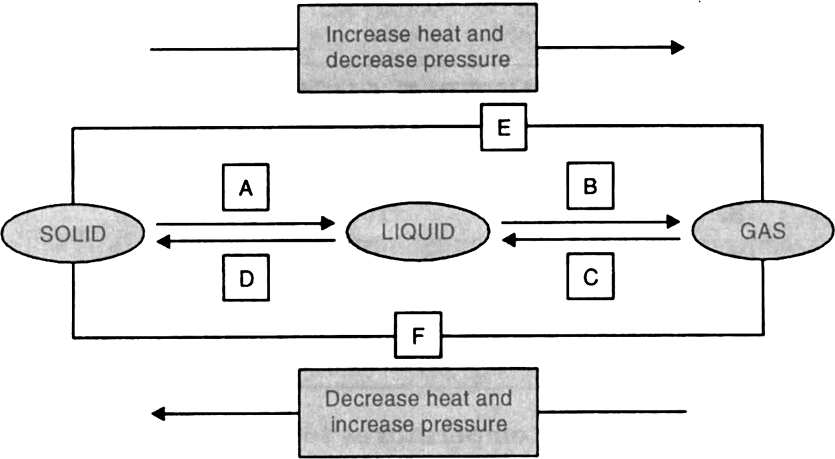
Give reasons:
A gas fills completely the vessel in which it is kept.
This is because the gases have large inter-particle spaces and the inter-particle attraction force is very weak. The gaseous particle can move freely into the empty spaces of another gas as particles of gas have very high kinetic energy. This property of gases is called diffusion. A gas can mix with any other gas easily and move rapidly from one part of vessel to another part of vessel. Therefore, gas particles can spread easily and fill the whole vessel.