Briefly describe the nitrogen cycle in the environment.
Or
Draw a neat labelled diagram to show nitrogen cycle in nature.
Nitrogen exists as free nitrogen in the atmosphere. In air N2 is about 78%. This free nitrogen is fixed into compounds of ammonia and nitrates. Most of the organisms cannot utilize nitrogen as molecular nitrogen.
Fixation of Nitrogen. Fixation of free nitrogen into compounds takes place by following means :
(a) Certain blue green algae and bacteria can fix atmospheric nitrogen.
(b) Nitrogen—fixing bacteria found in the nodules of roots of legumes such as gram, bean, pulses etc., fix atmospheric nitrogen into nitrogen containing compounds.
(c) Lightening also helps in the formation of nitrogen containing compounds.
Nitrogen containing fertilizers produced artificially in factories are the fixed form of nitrogen.
Plants take compounds containing nitrogen from the soil. Form plants nitrogen passes into food web. Decay of dead plants, animals and excreta like urine, faeces, causes return of nitrogen compounds to the soil. Denitrifying bacteria and fire cause liberation of free nitrogen in the atmosphere.
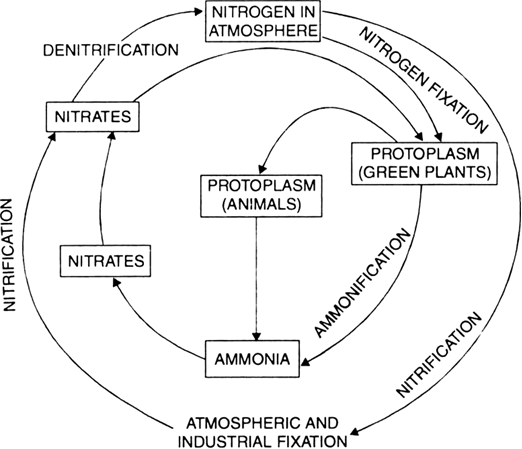
Nitrogen cycle.
Importance of Nitrogen Cycle. Nitrogen is an important constituent of tissues, proteins, enzymes, nucleic acids, amino acids. Atmosphere contains about 78 per cent nitrogen but plants and animals cannot use nitrogen in this form. Plants take nitrogen in the form of nitrates—the usable form. From plants, through food nitrogen travels to animals. If nitrogen in the form of proteins, amino acids, enzymes etc. remains locked up in the bodies of organisms, there will be shortage of usable form of nitrogen. Therefore, circulation of nitrogen in nature is very essential.



