Define the term—Element. Give an illustration in support of your definition.
Element: An element is a substance which contains same kind of atom. It cannot be converted into anything visibly simpler than itself. For example, element mercury is composed of only one kind of atoms (with atomic no. 80). Gold, copper, hydrogen are other examples of elements. After the discovery of isotopes, the definition of an element is modified. It is more correct to say that element is a substance made up of atoms, all having the same atomic number.
ILLUSTRATION
Take about 5.0 g of mercuric (II) oxide (red oxide of mercury) in a hard glass test tube fitted with cork and a delivery tube as shown in Fig. 2.10. Heat the tube over a bunsen flame, first slowly and then strongly. After sometime you would observe a gas coming out of the capillary tube. If you bring a glowing matchstick near the mouth of the tube, the stick starts burning with a flame. That is due to evolved oxygen which is supporter of combustion. Continue heating the glass tube. After sometime, no more gas is evolved and a shining liquid is left at the bottom of the glass tube. That is mercury.
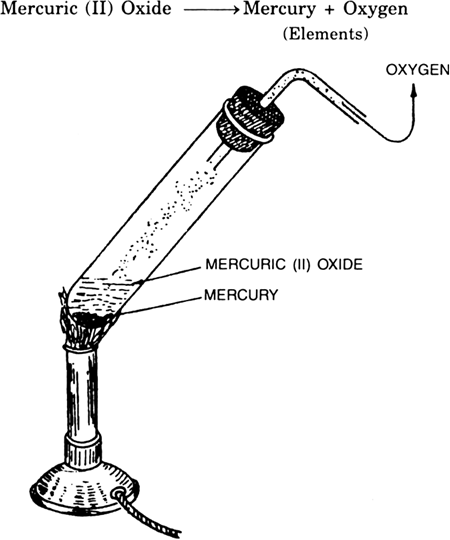
Conclusion. Red mercuric oxide on heating breaks up into two simpler substances, mercury and oxygen which are not further broken. Thus mercury and oxygen are elements.



