Define and explain distillation.
Distillation is a process to obtain pure liquid from its solution. Distillation can be defined as the conversion of impure liquid into vapours by evaporation and then condensation (cooling) of the vapours into the pure liquid.
Impure liquid or mixture is taken in a flask, fitted with a condenser as shown in Fig. When heated, liquid gets evaporated and the vapours pass to the delivery tube where they condense into liquid. The pure condensed liquid is collected in receiver. The solid remains behind in the flask, hence gets separated. This method is generally used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.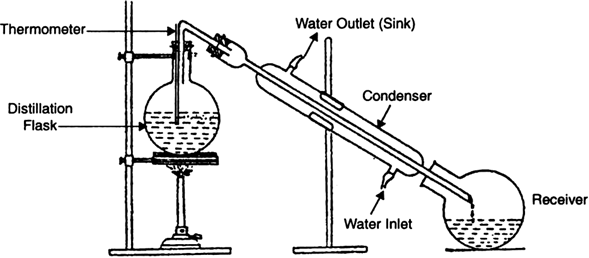
Fig. Distillation.



