Change the following temperatures to Kelvin scale.
1) 40°C
2) 80°C
3) 213°C
1) 40°C = 40 +273 = 313K
2) 80°C = 80 +273 =353K
3) 213°C = 213 +273=486K
Change the following temperatures to Kelvin scale.
1) 40°C
2) 80°C
3) 213°C
Give reason for the following observations.
We can get the smell of perfume sitting several metres away.
What is the physical state of water at
a) 25°C
b)00C
c)1000C
What is the physical state of water at
0°C
What is the physical state of water at
100°C
Give two reasons to justify
Water at room temperature is a liquid.
Give two reasons to justify
An iron almirah is a solid at room temperature.
Why is ice at 273K more effective in cooling than water at the same temperature ?
What produces more severe burns, boiling water or steam ?
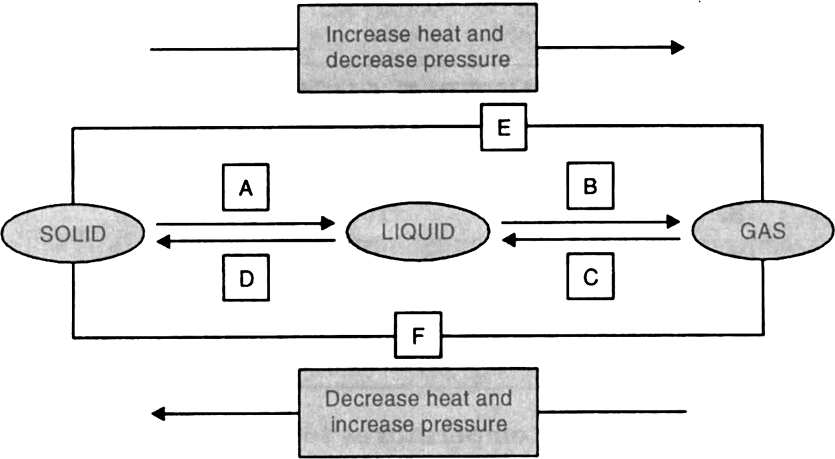
8. Name A, B, C, D, E and F in the following diagram showing state change :
Both boiling and evaporation convert a liquid into vapour. What is the difference between the two process
Mock Test Series