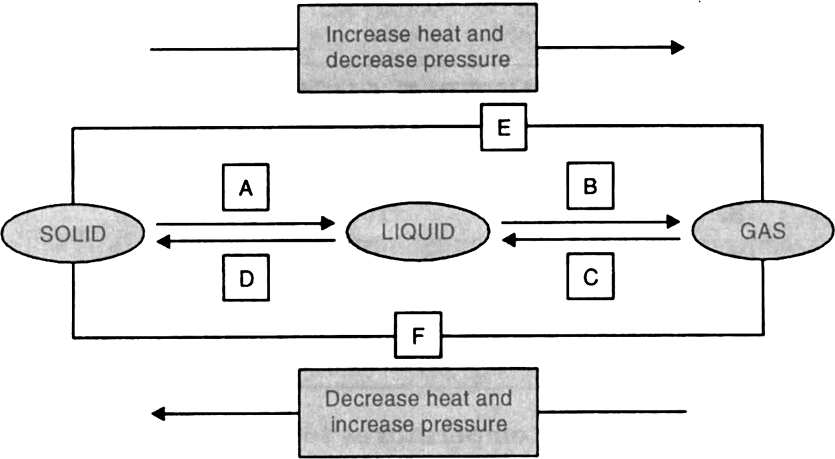
What is the relation between boiling point of a liquid and the molecular forces of attraction between the particles of a liquid?
Boiling point of liquid depend on the intermolecular force, Stronger the intermolecular forces of attraction, higher would be the boiling point. For example, particles of alcohol have weaker intermolecular forces of attraction than of water particles. This is in conformity with the fact that alcohol boils at lower temperature, of 78°C as compared to boiling point of water which is 100°C.