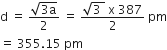Question
AB crystallises in a body centred cubic lattice with edge length 'a' equal to 387 pm. The distance between two oppositely charged ion in the lattice is
-
335 pm
-
250 pm
-
200 pm
-
300 pm
Solution
A.
335 pm
For body centred cubic (bcc) lattice, distance between two oppositely charged ions,