Question
Which of the following will most stable diazonium salt RN2+X-?
-
CH3N2+X-
-
C6H5N2+X-
-
CH3CH2N2+X-
-
C6H5CH2N2+X-
Solution
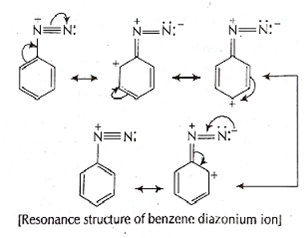
B.
C6H5N2+X-
Diazonium salt containing aryl group directly linked to the nitrogen atom is most stable due to resonance stabilization between the benzene nucleus and N-atom.