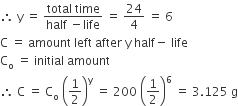Question
The half-life of a radioisotope is four hours. If the initial mass of the isotope was 200 g, the mass remaining after 24 hours undecayed is
-
1.042 g
-
4.167 g
-
3.125 g
-
2.084 g
Solution
C.
3.125 g
If y = number of half-lives