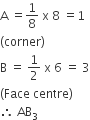Question
An ionic compound has a unit cell consisting of A ions at the corners of a cube and B ions on the centres of the faces of the cube. The empirical formula for this compound would be
-
A2B
-
AB
-
AB3
-
A3B
Solution
C.
AB3