Sponsor Area
Chemical Kinetics
Question
The time for half life period of a certain reaction A → Products is 1 hour. When the initial concentration of the reactant ‘A’ is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1 if it is a zero order reaction?
-
1 h
-
4 h
-
0.25 h
-
0.5 h
Solution
C.
0.25 h
Given that [A]o = 2 mol L-1
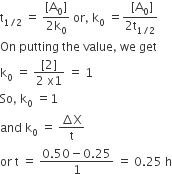
Some More Questions From Chemical Kinetics Chapter
The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y?
What will be the effect of temperature on rate constant?
A reaction is 50% complete in 2 hours and 75% complete in 4 hours. What is the order of the reaction.
Sponsor Area
Mock Test Series
Mock Test Series





