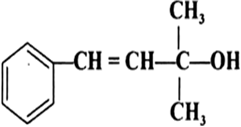
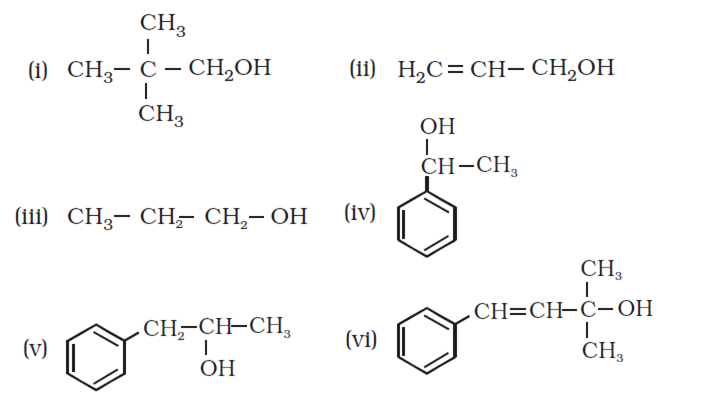
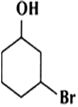
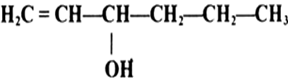
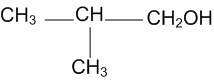
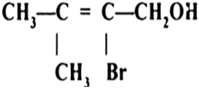Arrange the following compounds in order of decreasing acidity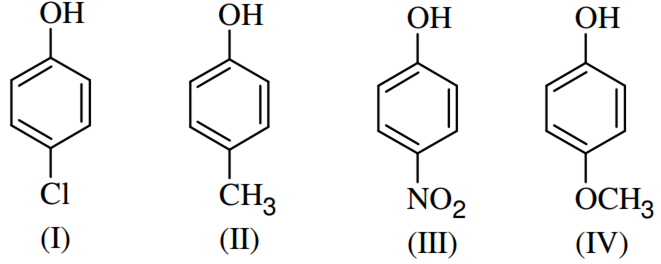
-
II>IV>I>III
-
I>II>III>IV
-
III>I>II>IV
-
IV>III>I>II
C.
III>I>II>IV
Electron withdrawing group increases the acidic strength of the compounds by destabilising and stabilising the phenoxide ion formed respectively.
The presence of an electron withdrawing group increases the acidic strength by stabilising the phenoxide ion. On other hands, the presence of electron releasing group destabilises the phenoxide ion and decrease the acidic strength.
NO2>Cl>CH3>OCH3