Sponsor Area
Solutions
Question
The ratio of masses of oxygen and nitrogen of a particular gaseous mixture is 1:4. The ratio of number of their molecule is
-
1:4
-
7:32
-
1:8
-
3:16
Solution
B.
7:32
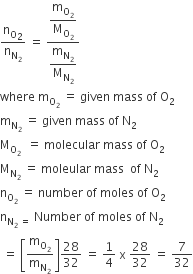
Some More Questions From Solutions Chapter
Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Sponsor Area
Mock Test Series
Mock Test Series





