Complete the following chemical equations:
![]()
Or
State reasons for the following:
(i) Cu (I) ion is not stable in an aqueous solution.
(ii) Unlike Cr3+, Mn2+, Fe3+ and the subsequent other M2+ ions of the 3d series of elements, the 4d and the 5d series metals generally do not form stable cationic species.
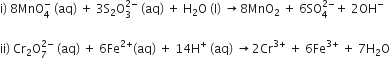
Or
(i) In aqueous solution, Cu+ ion undergoes oxidation to Cu2+ ion. The relative stability of different oxidation states can be seen from their electrode potentials.
Cu+(aq) + e- --> Cu(s) , E0red = 0.52V
Cu2+(aq) + 2e- ---> Cu(s), E0red = 0.34V
Thus overall reaction is :
2Cu+ (aq) ---> Cu2+ (aq) + Cu(s)
Due to more reduction electrode potential value of Cu+, it undergoes oxidation reaction quite feasibly. Hence, copper (I) ion is not stable in aqueous solution.
(ii) The valence shell electronic configurations of Cr3+, Mn2+, Fe3+ are d3, d5 and d3respectively. Owing to the symmetrical distribution of the d electrons, these ions can form stable cationic complexes. The atomic radii of the 4d and 5d transition elements are more than that of the 3d series elements. Hence, generally, they do not form stable cationic species.





