Describe the principle controlling each of the following processes:
(i) Preparation of cast iron form pig iron.
(ii) Preparation of pure alumina (Al2O3) from bauxite ore.(i) The iron obtained from blast furnaces is known as pig iron. It contains around 4%carbon and many impurities such as S, P, Si, and Mn in smaller amounts.
Cast iron is obtained by melting pig iron and coke using a hot air blast. It contains a lower amount of carbon (3%) than pig iron; cast iron is extremely hard and brittle.
(ii) Bauxite usually contains silica, iron oxide, and titanium oxide as impurities. In the process of leaching, alumina is concentrated by digesting the powdered ore with a concentrated solution of NaOH at 473-523 K and 35-36 bar. Under these conditions, alumina (Al2O3) dissolves as sodium meta-aluminate and silica (SiO2) dissolves as sodium silicate leaving the impurities behind.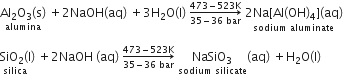
The impurities are then filtered and the solution is neutralized by passing CO2 gas. In this process, hydrated Al2O3 gets precipitated and sodium silicate remains in the solution. Precipitation is induced by seeding the solution with freshly prepared samples of hydrated Al2O3.
![]()
Hydrated alumina thus obtained is filtered, dried, and heated to give back pure alumina (Al2O3).
![]()





