Sponsor Area
Alcohols, Phenols And Ethers
Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol.
Some reactive alkenes undergo direct hydration in the presence of mineral acids which act as catalysts. The addition of water to the double bond takes place in accordance with Markonikoff’s rule.
The mechanism of reaction involves the following three steps:
(i) Protonation of alkene to form carbocation by electrophilic attack of H3O+.
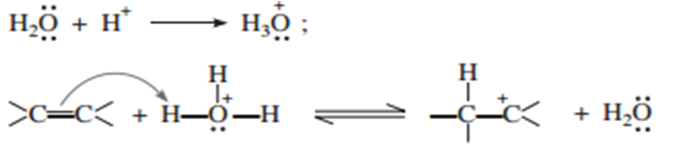
(ii) Nucleophilic attack of water of carbocation.
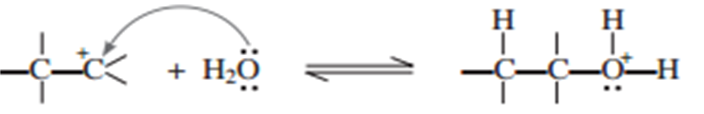
(iii) Deprotonation to from an alcohol.

Some More Questions From Alcohols, Phenols and Ethers Chapter
Name the following compounds according to IUPAC system.
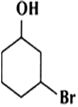
Name the following compounds according to IUPAC system.
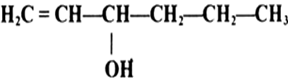
Name the following compounds according to IUPAC system.
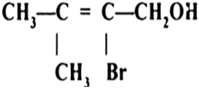
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?
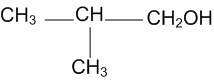

Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
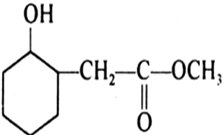
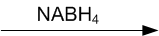
Write structures of the products of the following reactions:
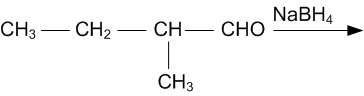
Give structures of the products you would expect when each of the following alcohol reacts with,
(a) HCl—ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol (ii) 2-Methyl butan-2-ol.
Sponsor Area
Mock Test Series
Mock Test Series





