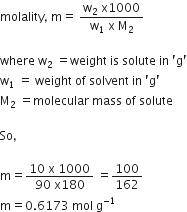(a) Define the following terms:
(i) Ideal solution
(ii) Azeotrope
(iii) Osmotic pressure
(b) A solution of glucose (C6H12O6) in water is labeled as 10% by weight. What would be the molality of the solution?
(Molar mass of glucose = 180 g mol-1)
(a)
(i) Ideal Solution:
Solutions which obey Raoult’s law over the entire range of concentrations are known as the ideal solution. Along with that for ideal solution:
Enthalpy of mixing of the pure components to form the solution i.e  mix H = 0 and volume of mixing,
mix H = 0 and volume of mixing,  mix V = 0.
mix V = 0.
An ideal solution will be formed when intermolecular forces of attraction between the molecules of solute (A - A) and those between the molecules of solvent (B -B) are nearly equal to those between solute and solvent molecules (A - B).
For Example n-Hexane and n-heptane
(ii) Azeotropes
Binary mixtures which have the same composition in liquid and vapour phase, and have constant boiling points are known as azeotropes. It is not possible to separate its components by fractional distillation .There are two types of azeotropes:
Minimum boiling azeotrope, example: Ethanol-water mixture containing ethanol approximately 95% by volume.
Maximum boiling azeotrope, example: Nitric acid-water mixture containing 68% nitric acid and 32% water by mass.
(iii) Osmotic Pressure
The process of flow of solvent molecules from pure solvent to a solution or from a solution of lower concentration to a solution of higher concentration through a semi-permeable membrane is called osmosis. The pressure required to prevent the flow of solvent due to osmosis is called osmotic pressure (Ï€) of the solution.
Osmotic pressure is directly proportional to the molarity C of the solution at a given temperature T.
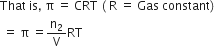
Where,
n2 = Number of moles of solute
V= Volume of the solution in litres
(b) Assume that 100 g of solution contains 10 g of glucose and 90 g of water as our glucose solution is 10% by weight
Where w2 = weight is solute in 'g'
w1 = weight of solvent in 'g'
M2 = Molecular mass of solute.
So,