Sponsor Area
Chemical Kinetics
Question
(a) For a reaction A + B --> P, the rate law is given by,
r = k [A]1/2 [B]2.
What is the order of this reaction?
(b) A first order reaction is found to have a rate constant k = 5·5 x 10-14 s-1. Find the half-life of the reaction.
Solution
(a)
For A + B--> P
r = k [A]1/2 [B]2
The order of the reaction = ![]()
(b) For first order reaction
k = 5.5 x 10-14 s-1
Half-life period ( ![]() ) for the first order reaction
) for the first order reaction
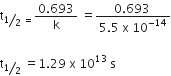
Some More Questions From Chemical Kinetics Chapter
What will be the effect of temperature on rate constant?
A reaction is 50% complete in 2 hours and 75% complete in 4 hours. What is the order of the reaction.
The rate law for the decomposition of N2O5 is rate = k[N2O5]. What is the significance of k in this equation?
Define activation energy of a reaction.
Sponsor Area
Mock Test Series
Mock Test Series





