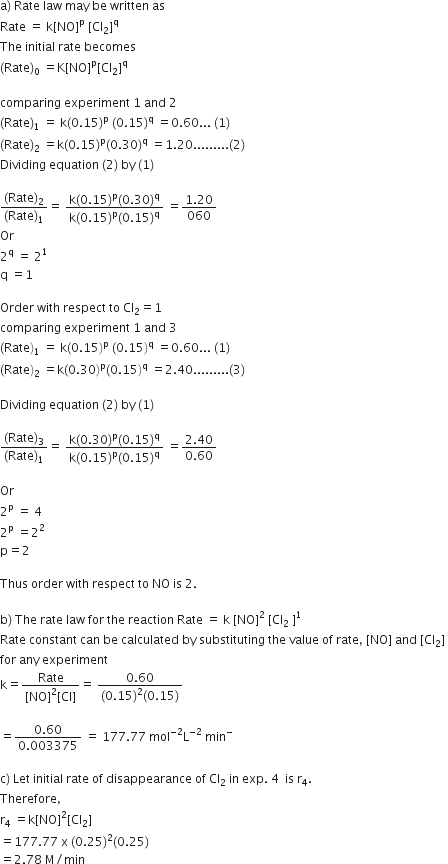Question
For the reaction
2NO(g) + Cl2(g) --> 2NOCl(g)
The following data were collected. All the measurements were taken at 263 K:
|
Experiment No. |
Initial [NO] (M) |
Initial [Cl2] (M) |
Initial rate of disappearance of Cl2(M/min) |
|
1 |
0.15 |
0.15 |
0.60 |
|
2 |
0.15 |
0.30 |
1.20 |
|
3 |
0.30 |
0.15 |
2.40 |
|
4 |
0.25 |
0.25 |
? |
(a) Write the expression for rate law.
(b) Calculate the value of rate constant and specify its units.
(c) What is the initial rate of disappearance of Cl2 in exp. 4?
Solution