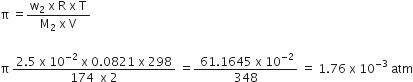Question
Determine the osmotic pressure of a solution prepared by dissolving 2.5 x 10-2 g of K2SO4 in 2L of water at 250C, assuming that it is completely dissociated.
(R = 0.0821 L atm K-1 mol-1, Molar mass of K2SO4 = 174 g mol-1)
Solution
w2 = 2.5 x 10-2 g (Mass of K2SO4)
M2 = 174 g mol-1 (Molar mass of K2SO4)
V = 2L,
R = 0.0821 L atm K-1 mol-1 and
T = 25°C = 298 K
Osmotic pressure,