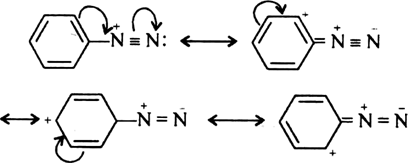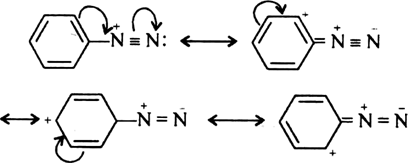Question
Why are aryl diazonium ions more stable than alkyl diazonium ions?
Solution
Aryl diazonium ions  are more stable than R-N2+ due to resonance. Aryl diazonium ions have more contributing structure than alkyl diazonium ions. Hence, aryl diazonium is more stable.
are more stable than R-N2+ due to resonance. Aryl diazonium ions have more contributing structure than alkyl diazonium ions. Hence, aryl diazonium is more stable.