Question
Account for the fact that:
p-nitroaniline is a weak base.
Solution
There is an important resonance interaction between the amino and nitro groups in p-nitroaniline as shown.
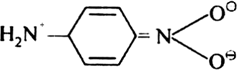
Because of the contribution of this structure to the actual state of the molecule, there is a greater loss of resonance energy when p-nitro aniline is converted to its conjugate base p-NH3.C6H4.NO2 than when aniline is converted to the anilinium ion, NH3+C6H5. Hence, p-nitroaniline is a weaker base than aniline which itself is a weak base.
Because of the contribution of this structure to the actual state of the molecule, there is a greater loss of resonance energy when p-nitro aniline is converted to its conjugate base p-NH3.C6H4.NO2 than when aniline is converted to the anilinium ion, NH3+C6H5. Hence, p-nitroaniline is a weaker base than aniline which itself is a weak base.



