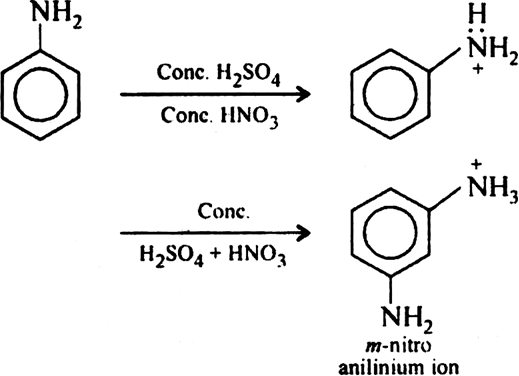
Question
Explain what happens when aniline reacts with a mixture of sulphuric acid and nitric acid?
Solution
Being a strong oxidising agent, HNO3 oxides aniline into tert oxidation product on treating it with conc. H2SO4 and HNO3. However, under controlled conditions, m-nitroaniline is formed along with some amount of p-nitroaniline. This is due to the reason that under strongly acidic conditions — NH, a group of aniline is converting into —NH3+(anilinium ion) which is m-directing, thus the major product is m-nitroaniline.