Question
Give plausible explanation for each of the following:
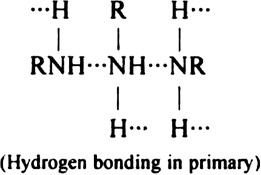
Why are primary amines higher boiling than tertiary amines?
Solution
Amines are polar compounds and can form H-bonds as proton acceptors. Only primary and secondary amines can form intermolecular H-bonding due to the presence of H atom at N atom. Tertiary amines cannot form intermolecular in-bonding. Primary amines are higher boiling due to the presence of a stronger intermolecular force of attraction due to H-bonding.