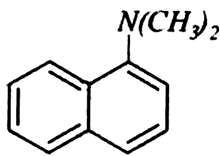
Question
Why are aromatic amines weaker bases than aliphatic amines?
Solution
The phenyl group (C6H5) withdraws electron density from the nitrogen atom and this makes aromatic amine a weak base (mesomeric effect).