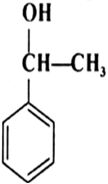
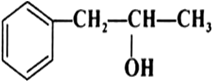
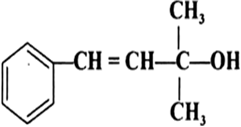
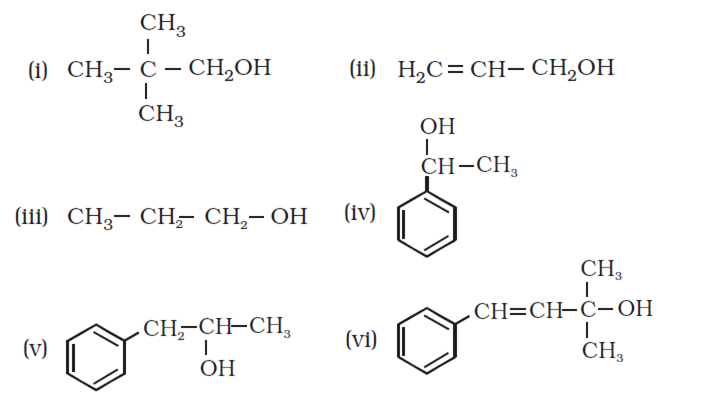
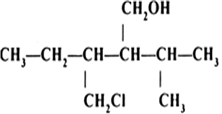
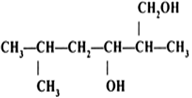
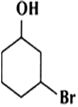
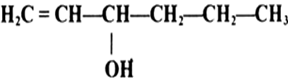
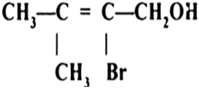Question
Phenol is acidic but does not decompose NaHCO3 solution while carbonic acids like CH3COOH decompose NaHCO3 solution. Why?
Solution
Phenol is weaker acid than carbonic acids, Ka value of phenol is very less than that of carbonic acids like CH3COOH, C6H5COOH etc. Therefore phenol does not decompose NaHCO3 solution.