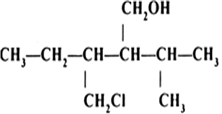
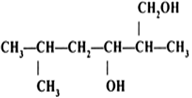
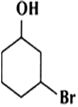
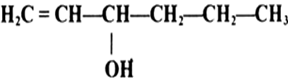
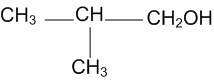
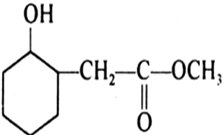
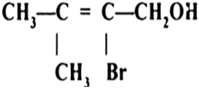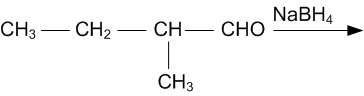Question
Why phenol is stronger acid than ethanol?
Solution
Acidity of phenols: The reactions of phenol with metals (e.g., sodium, aluminium) and sodium hydroxide indicate its acidic nature. The hydroxyl group, in phenol is directly attached to the sp2 hybridised carbon of benzene ring which acts as an
electron withdrawing group. Due to this, the charge distribution in phenol molecule, as depicted in its resonance structures, causes the oxygen of –OH group to be positive.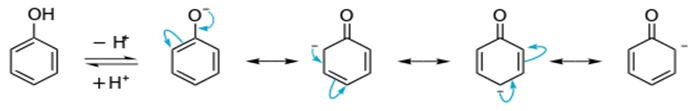
In case of ethanol, no such resonace structure is shown as phenol. Hence, phenol is stronger acid than ethanol.