Question
Calculate (a) molality (b) molarity and (c) mole fraction of KI if the density of 20% (mass/mass) aqueous KI is 1.202 g mL-1.
Solution
(a) 20% (mass/mass) means that 20 g of KI is present in 80 g of water.
Therefore, Moles of KI in solution

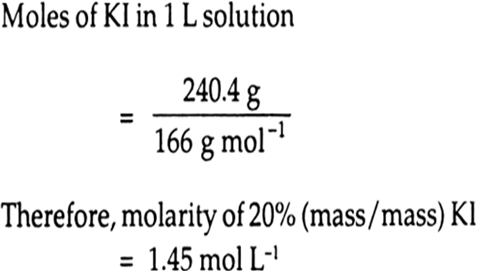
moles of KI = 20/166 =0.12mol
moles of water =80/18 =4.44mol
therefore, mole fraction of KI
=
Therefore, Moles of KI in solution

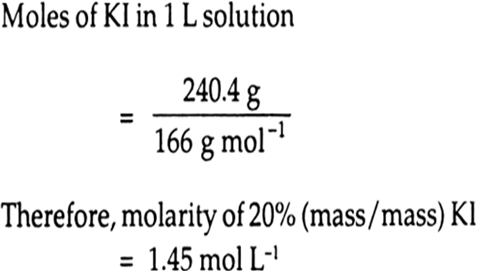
moles of KI = 20/166 =0.12mol
moles of water =80/18 =4.44mol
therefore, mole fraction of KI
=



