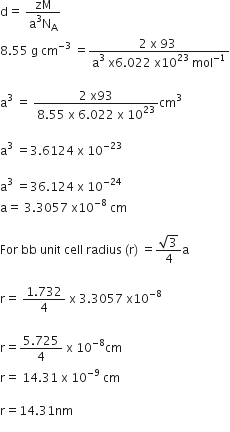Question
Niobium crystallises in body centered cubic structure. If density is 8.55 g cm–3, calculate atomic radius of niobium using its atomic mass 93 u.
Solution
We have give that,
Density (d)=8.55 g Cm-3
Atomic mass (M) =93u =93 gMol-1
Atomic radius (r) = ?
We know that, Avogadro number Na =6.022 x 10 23 mol-1
since given lattice is bcc
therefore
Number of atoms per unit cell (z) =2
we know that