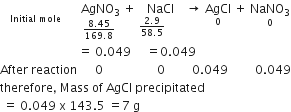What is the mass of precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8% NaCl solution?
(Ag = 107.8, N = 14, O = 16, Na = 23, Cl = 35.5)
-
28 g
-
3.5 g
-
7 g
-
14 g
C.
7 g
For the calculation of the mass of AgCl precipitate, we find the mass of AgNO3 and NaCl in equal volume with the help of mole concept. 16.9% solution of AgNO3 means 16.9 g AgNO3 is present in 100 mL solution.
therefore, 8.45 g AgNO3 will present in 50 mL solution similarly,
5.8 g NaCl is present in 100 ml solution