The formation of the oxide ion O2- (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below,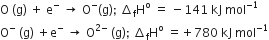
Thus, the process of formation of O2- in the gas phase is unfavourable even though O2- is isoelectronic with neon. It is due to the fact that
-
electron repulsion outweighs the stability gained by achieving a noble gas configuration
-
O- ion has comparatively smaller size that oxygen atom
-
oxygen is more electronegative
-
the addition of electron in oxygen result in the large size of the ion
A.
electron repulsion outweighs the stability gained by achieving a noble gas configuration
Electron repulsion predominates over the stability gained by achieving noble gas configuration. Hence, the formation of O2- in the gas phase is unfavourable.



