Which of the following lines correctly show the temperature dependence of equilibrium constant K, for an exothermic reaction?
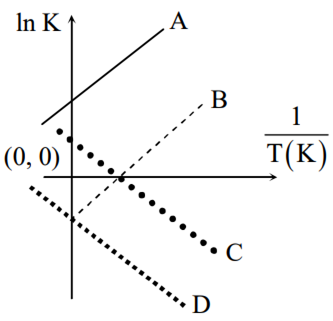
A and D
A and B
B and C
C and D
B.
A and B
Therefore ln K vs 1/T the graph will be a straight line with slope equal to .Since reaction is
exothermic, therefore itself will be negative resulting in positive slope.



