Sponsor Area
Equilibrium
Question
The equilibrium constant for the reaction N2(g) + O2(g) ⇌ 2NO(g) at temperature T is 4×10-4. The value of Kc for the reaction NO(g) ⇌ 1/2N2(g) + 1/2O2(g) at the same temperature.
-
2.5×102
-
0.02
-
4 x 10-4
-
50
Solution
D.
50
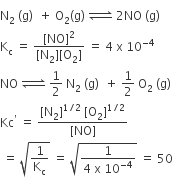
Some More Questions From Equilibrium Chapter
What is equilibrium?
Give two examples of physical equilibrium.
What kind of molecules in a liquid can evaporate?
Name the factors on which vapour pressure of any liquid depends.
Which measurable property becomes constant in water vapour equilibrium?
Give an example from daily life in which there is gas solution equilibrium?
A crystal of common salt of a given mass is kept in its aqueous solution. After 24 hours, its mass remains the same. Is the crystal in equilibrium with the solution?
Sponsor Area
Mock Test Series
Mock Test Series





