Question
The conjugate base of H2PO4- is
-
PO43-
-
HPO42-
-
H3PO4
-
P2O5
Solution
B.
HPO42-
H3PO4 is a tribasic acid, thus ionising in three steps.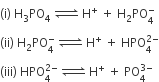
The conjugate base is formed when an acid loses its proton. Thus, HPO42- is the conjugate base of H2PO4- (which is an acid in step II, but is the conjugate base of H3PO4 in step I)



