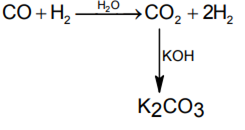
In context with the industrial preparation of hydrogen from water gas (CO + H2), which of the following is the correct statement?
-
CO and H2 are fractionally separated using differences in their densities
-
CO is removed by absorption in aqueous Cu2Cl2 solution
-
H2 is removed through occlusion with Pd
-
CO is oxidised to CO2 with steam in the presence of a catalyst followed by absorption of CO2 in alkali
D.
CO is oxidised to CO2 with steam in the presence of a catalyst followed by absorption of CO2 in alkali