The ionization enthalpy of a hydrogen atom is 1.312 × 106Jmol−1. The energy required to excite the electron in the atom from n = 1 to n = 2 is
-
8.51 × 105 Jmol−1
-
6.56 × 105 Jmol−1
-
7.56 × 105 Jmol−1
-
9.84 × 105 Jmol−1
D.
9.84 × 105 Jmol−1
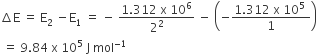
The ionization enthalpy of a hydrogen atom is 1.312 × 106Jmol−1. The energy required to excite the electron in the atom from n = 1 to n = 2 is
8.51 × 105 Jmol−1
6.56 × 105 Jmol−1
7.56 × 105 Jmol−1
9.84 × 105 Jmol−1
D.
9.84 × 105 Jmol−1
What is the name of the remaining part of atom except outer orbit?
Name the particles which determine the mass of an element.
What are α-particles?
What are the fundamental particles present in a neutral atom having atomic number greater than 1?
Do protons and neutrons have identical mass?
When α-particles are sent through a thin metal foil, most of them go straight through the foil. What inference do you draw from it?
What did Rutherford's experiment on scattering of particles show for the first time?
What is Plum-Pudding model of the atom?
Mock Test Series