Standard entropy of X2, Y2 and XY3 are 60, 40 and 50 JK−1
mol −1, respectively. For the reaction,1/2X2 + 3/2Y2, ΔH = -30 kJ,to be at equilibrium, the temperature will be
-
1250 K
-
500 K
-
750 K
-
1000 K
C.
750 K
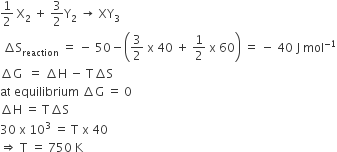
Standard entropy of X2, Y2 and XY3 are 60, 40 and 50 JK−1
mol −1, respectively. For the reaction,1/2X2 + 3/2Y2, ΔH = -30 kJ,to be at equilibrium, the temperature will be
1250 K
500 K
750 K
1000 K
C.
750 K
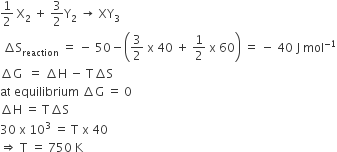
What do you understand by the terms: the system and surroundings?
Classify the following into different types of systems:
(i) Tea placed in a cup
(ii) Tea placed in a tea-pot
(iii) Tea placed in a thermosflask.
Define a closed system.
Define an isolated system.
Define intensive properties.
Define extensive properties.
Why all living systems need to be 'open systems'?
Define state variables of a system.
From thermodynamic point of view, to which system the animals and plants belong?
What is a state function?
Mock Test Series