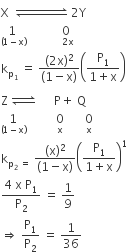Question
The equilibrium constants KP1 and KP2 for the reactions X ⇌2Y and Z ⇌ P + Q, respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressure at these equilibria is
-
1:1
-
1:36
-
1:9
-
1:3
Solution
A.
1:1