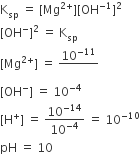Question
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?
-
9
-
10
-
11
-
8
Solution
B.
10