The solubility product of silver bromide is 5.0 x 10-13. The quantity of potassium bromide (molar mass taken as 120 g mol–1)to be added to 1 litre of 0.05 M solution of silver nitrate to start the precipitation of AgBr is
-
1.2 x 10-10 g
-
1.2 x 10-9 g
-
6.2 x 10-5 g
-
5.0 x 10-8 g
A.
1.2 x 10-10 g
Ksp of AgBr = [Ag+][Br-] = 5.0 x 10-13
[Ag+] = 0.05 M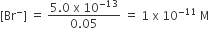
Moles of KBr = 1 x 10-11 x 1 = 1 x 10-11
weight of KBr 1 x 10-11 x 120 = 1.2 x 10-19 g



