-
Call Us
+91 8076753736 -
Send us an Email
[email protected]
Equilibrium
In aqueous solution the ionization constants for carbonic acid are
K1 = 4.2 x 10-7 and K2 = 4.8 x 10-11.
Select the correct statement for a saturated 0.034 M solution of the carbonic acid.
-
The concentration of CO32- is 0.034 M
-
The concentration of CO32- is greater than that of HCO3-
-
The concentration of H+ and HCO3- are approximately equal
-
The concentration of H+ is double that of CO32-
C.
The concentration of H+ and HCO3- are approximately equal
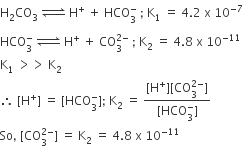
Second dissociation constant is much smaller than the first one. Just a small fraction of total HCO3- formed will undergo the second stage of ionization. Hence in a saturated solution.
Sponsor Area
Some More Questions From Equilibrium Chapter
What kind of molecules in a liquid can evaporate?
Name the factors on which vapour pressure of any liquid depends.
Which measurable property becomes constant in water vapour equilibrium?
Give an example from daily life in which there is gas solution equilibrium?
A crystal of common salt of a given mass is kept in its aqueous solution. After 24 hours, its mass remains the same. Is the crystal in equilibrium with the solution?
What is the role of forward and backward reactions at equilibrium of a reversible reaction?
Mock Test Series
Sponsor Area
 Wired Faculty
Wired Faculty



